Akanocure building blocks
Akanocure Pharmaceuticals Inc. Website: www.akanocure.com
Purdue Research Foundation News - February 7, 2017: Purdue-affiliated pharmaceutical company launches product to produce rare disease-fighting compounds
Introduction
Polypropionates are a group of natural products that represent an important class of metabolites in plants, bacteria, insects, fungi, and marine organisms. The group includes compounds that can inhibit the growth of bacteria, viruses, fungi, parasites, or human tumor cells.
There is a special interest in the polypropionates family of marine natural compounds because of their important biological activities. Polypropionates are considered a subclass of the polyketide family of natural products along with their biosynthetic congeners; aromatic polyketides and fatty acids (Fig. 1). Over 10,000 polyketide natural products have been discovered to date, of which an estimated 1% possess bioactive properties, more than 5x the number found in any other known family of natural products.
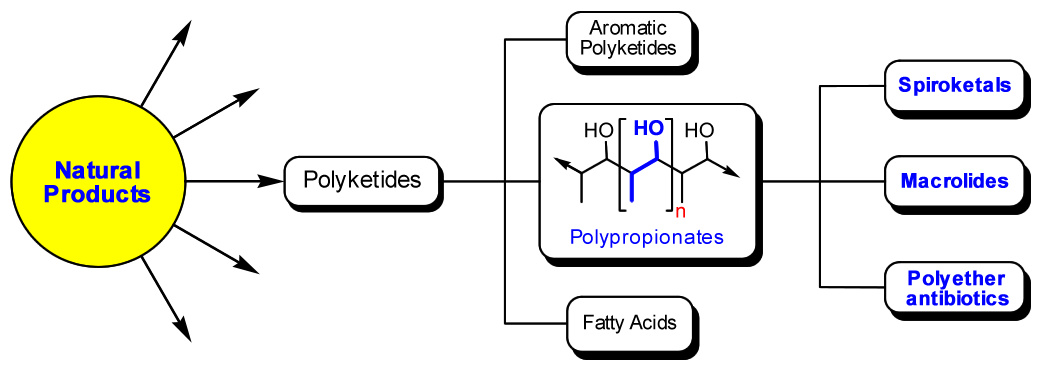
Figure 1. Subtypes of polyketide natural products (adapted from Stereoselective Synthesis of Drugs and Natural Products, by Vasyl Andrushko, Natalia Andrushko)
Polypropionates are represented by an aliphatic chain substituted with alternating methyl and hydroxyl groups. The polypropionate building blocks are either stereotriads (3 stereocenters and 8 possible members), stereotetrads (4 stereocenters and 16 possible members), stereopentads (5 stereocenters and 32 possible members), and so on.
The library of 16 stereotetrads is shown in Fig. 2. The fact that stereotetrad units contain four adjacent chiral carbon atoms that form a linear backbone has been a major synthetic challenge. The latter property, as well as their sometimes-unprecedented potencies and activities, has driven numerous efforts, including ours, toward their synthesis.
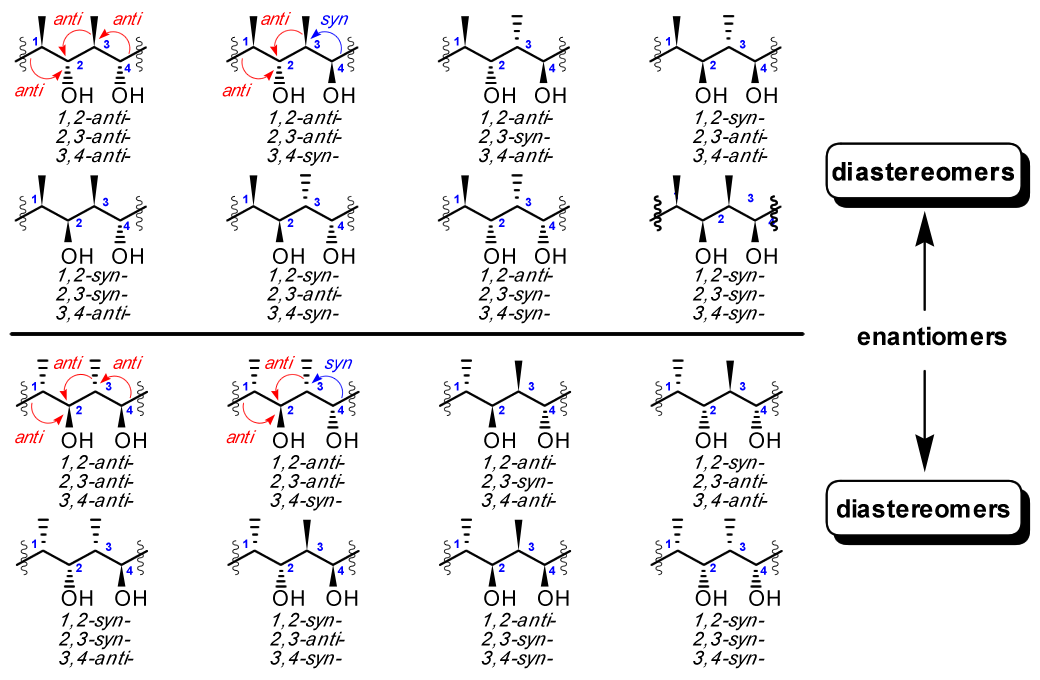
Figure 2. The family of stereotetrad fragments
Representative examples of polypropionate natural products are given in Fig. 3 showing the stereotetrad fragments that are part of the structure. The examples shown demonstrated unique biological activities as anti-bacterial, anti-fungal, and anti-tumor agents.
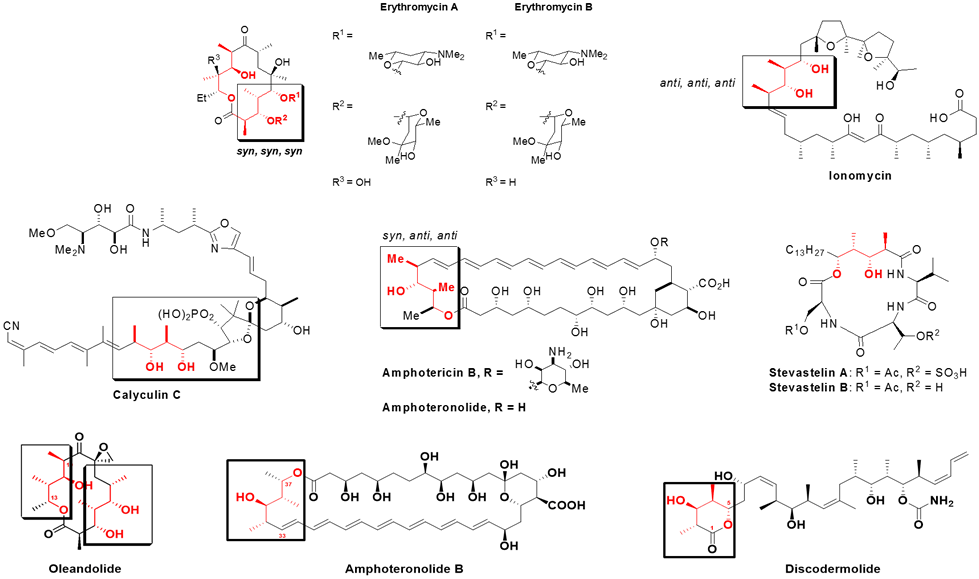
Figure 3. Representative examples of polypropionate natural products.
We provide all possible sixteen stereotetrads (in the form of chiral lactones) on practical scales as a prelude to total synthesis of some polypropionate natural products (Fig. 4).
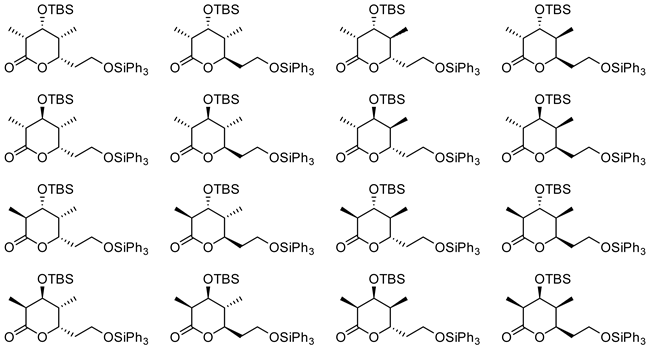
Figure 4. A library of chiral lactones (cyclic stereotetrads). These lactones can be opened by a nucleophile to afford termini differentiated linear stereotetrads.
Our library of enantiopure lactones possesses great synthetic versatility to further elaborate multiple versions of termini-differentiated linear segments ready for couplings as shown in the Fig. 5. The potential of those chiral lactones to serve as common precursors for multiple desired versions of linear segments bearing four contiguous stereocenters makes these lactones ideal tools and very useful building blocks for total synthesis of polyproprionate natural products. In addition, their synthetic flexibility enables them to be useful entries for more diverse and complex libraries.
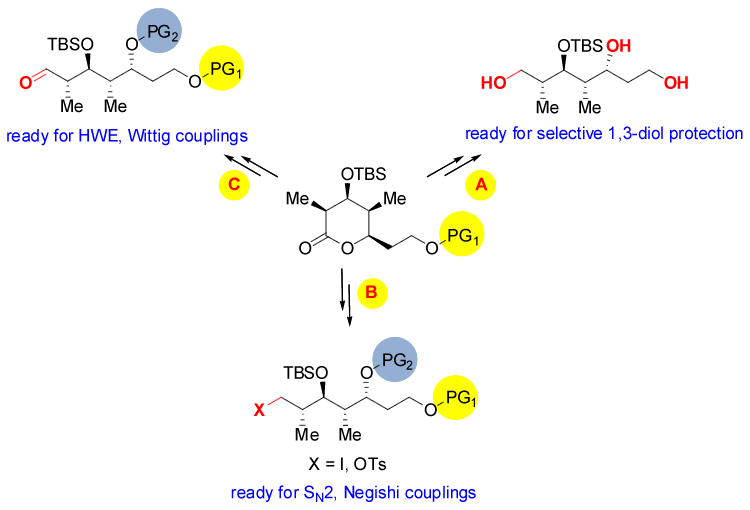
Figure 5.Synthetic versatility of the chiral lactones. (A) full reduction will elaborate the triol where the 1,2-diol moiety could be selectively protected leaving the primary alcohol end ready for selective manipulation, (B) Opening the lactone via (e.g. dimethyl amide formation) can lead, within a few steps, to the linear segment bearing an iodide or a tosylate that is ready for SN2 operations or Negishi type couplings, (C) Employing a Weinreb amine to open the lactone can ultimately lead to a linear aldehyde that can undergo multiple olefination protocols including Wittig, HWE, Julia-Kocienski and useful hydrazone formations.
These precursors can be used to generate interchangeable regional and conformational control elements that can be of great value in conjunction with in-silico drug docking calculations to assist rational drug design/SAR studies.
References
- For lactone openings with oxygen nucleophiles: (a) Hoye, T. R.; Jeon, J.; Kopel, L. C.; Ryba, T. D.; Tennakoon, M. A.; Wang, Y. Angew. Chem. Int. Ed. 2010, 49, 6151 (b) Xavier, G. Roulland, Emmanuel, R. Org. Lett. 2009, 11, 4700
- For lactone openings with primary nitrogen nucleophiles: (a) Metta-Magana, A. J.; Reyes-Martinez, R.; Tlahuext, H. Carbohydr. Res. 2007, 342, 243 (b) Dalby, S. M.; Goodwin-Tindall, J.; Paterson, I. Angew. Chem. Int. Ed. 2013, 52, 6517.
- For lactone openings with sulfur nucleophiles: Raghavan, S.; Vinoth Kumar, V. Org. Biomol. Chem. 2013, 11, 2847.
- For full lactone reductions: (a) Yadav, J. S.; Singh, V. K.; Srihari, P. Org. Lett. 2014, 16, 836 (b) Mineeva, I.V. Russ. J. Org Chem. 2015, 51, 1061.
 Akinalytics
Akinalytics Midwest GMP
Midwest GMP Polymer Blog
Polymer Blog









